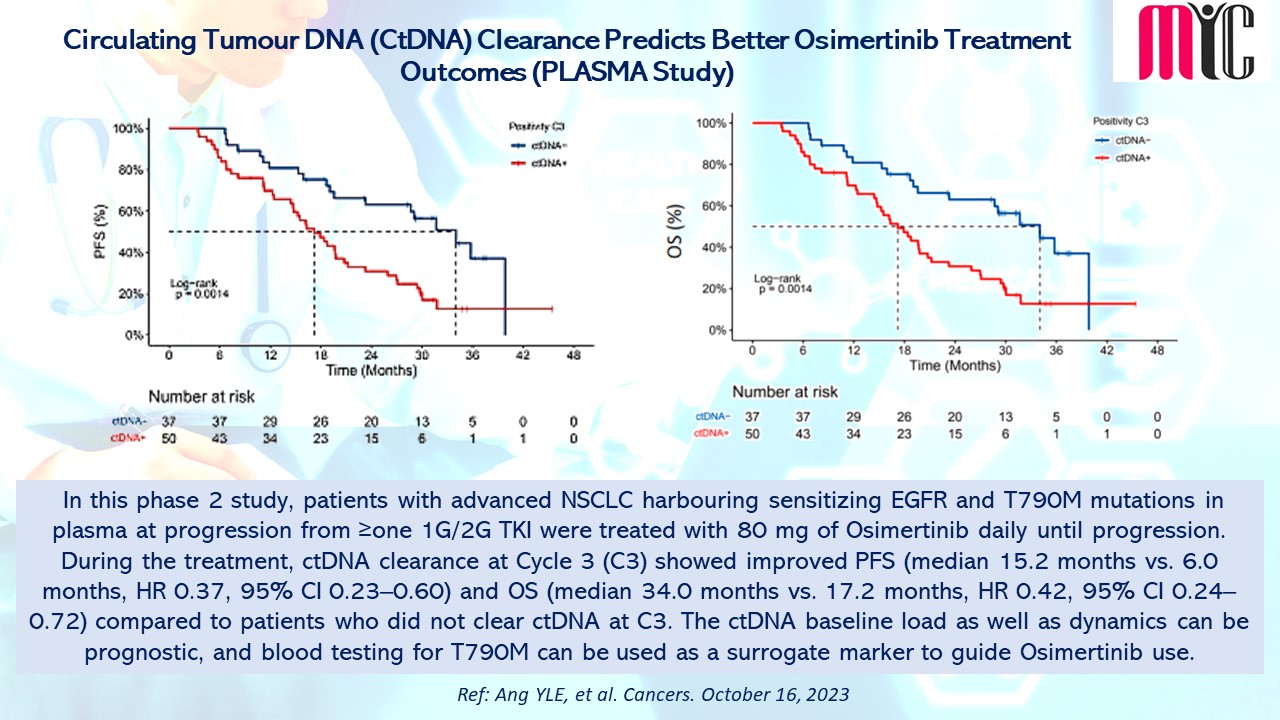
31 Oct Circulating Tumour DNA (CtDNA) Clearance Predicts Better Osimertinib Treatment Outcomes (PLASMA Study)
In this phase 2 study, patients with advanced NSCLC harbouring sensitizing EGFR and T790M mutations in plasma at progression from ≥one 1G/2G TKI were treated with 80 mg of Osimertinib daily until progression. During the treatment, ctDNA clearance at Cycle 3 (C3) showed improved PFS (median 15.2 months vs. 6.0 months, HR 0.37, 95% CI 0.23–0.60) and OS (median 34.0 months vs. 17.2 months, HR 0.42, 95% CI 0.24–0.72) compared to patients who did not clear ctDNA at C3. The ctDNA baseline load as well as dynamics can be prognostic, and blood testing for T790M can be used as a surrogate marker to guide Osimertinib use. (Ref: Ang YLE, et al. Cancers. October 16, 2023)
#oncologyresearch #clinicalresearch #clinicaldevelopment




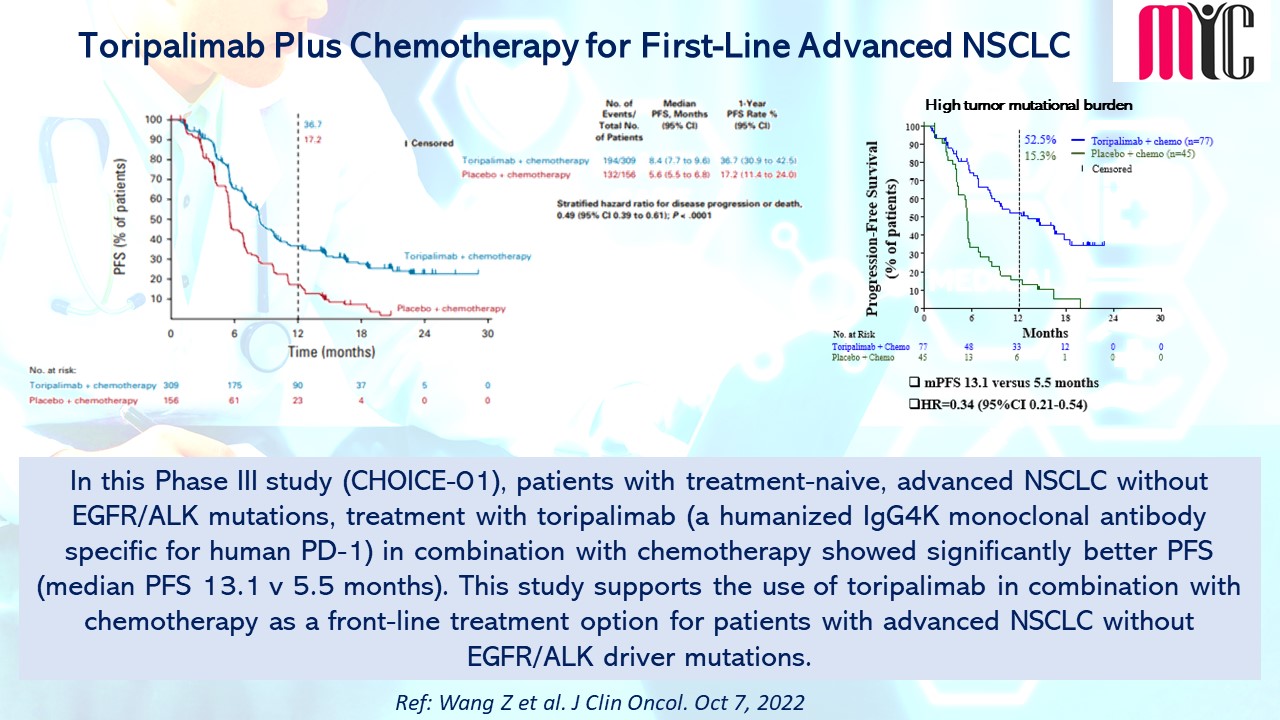
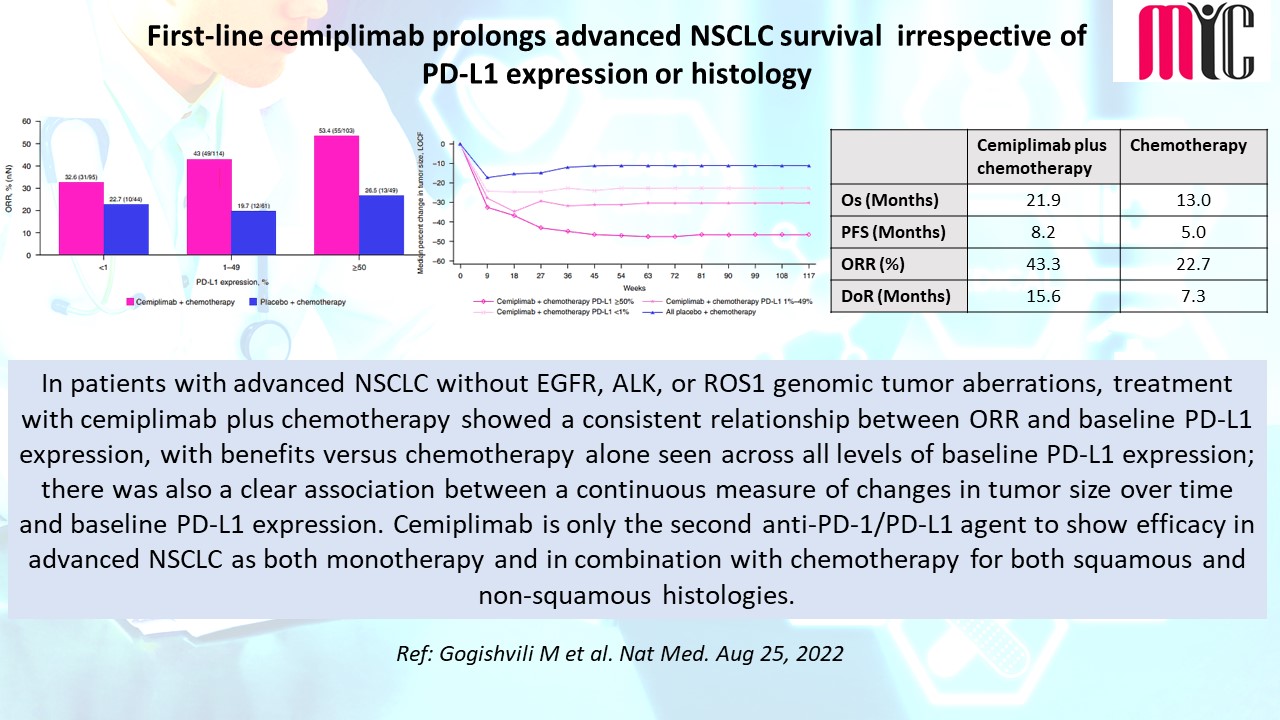
#oncologyresearch #clinicalresearch #clinicaldevelopment