29 Oct Toripalimab Plus Chemotherapy for First-Line Advanced NSCLC
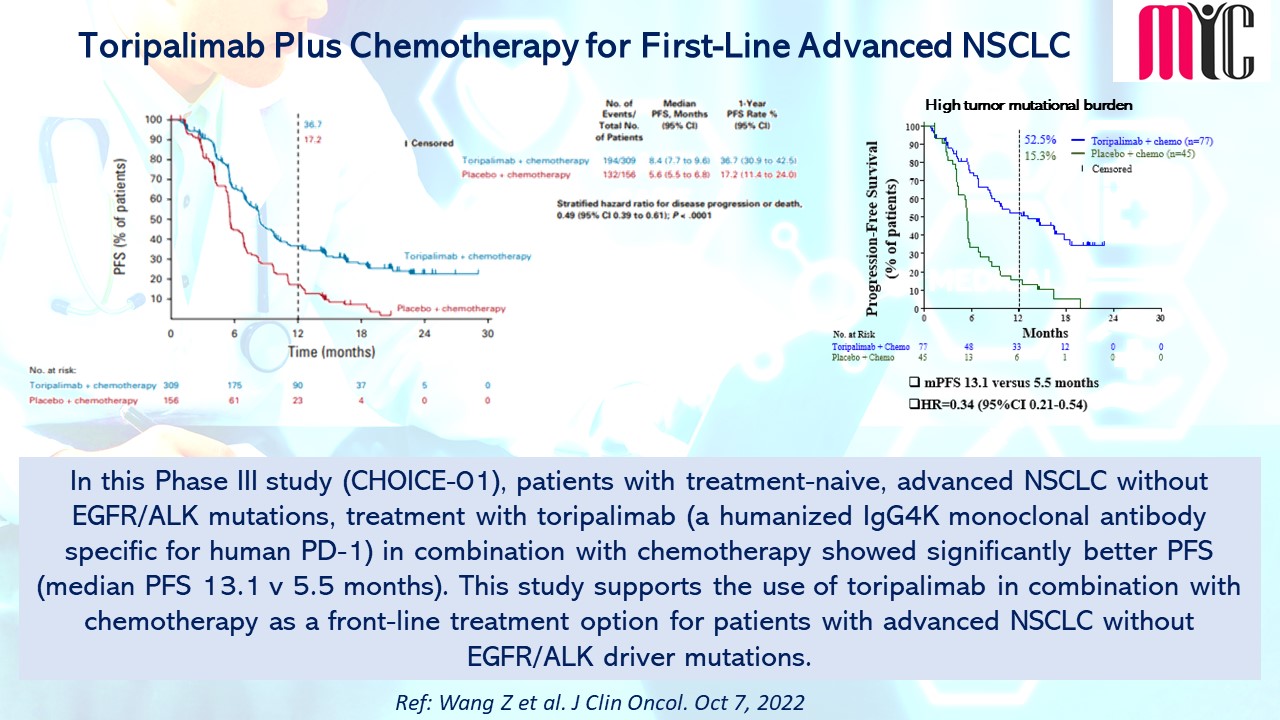
In this Phase III study (CHOICE-01), patients with treatment-naive, advanced NSCLC with EGFR/ALK mutations, treatment with toripalimab (a humanized IgG4K monoclonal antibody specific for human PD-1) in combination with chemotherapy showed significantly better PFS (median PFS 13.1 v 5.5 months). This study supports the use of toripalimab in combination with chemotherapy as a front-line treatment option for patients with advanced NSCLC without EGFR/ALK driver mutations. (Ref: Wang Z et al. J Clin Oncol. Oct 7, 2022)
#oncologyresearch #clinicalresearch #clinicaldevelopment
https://www.linkedin.com/feed/update/urn:li:activity:6992186328616472577/




Sorry, the comment form is closed at this time.