03 Jun Capivasertib in Hormone Receptor–Positive Advanced Breast Cancer
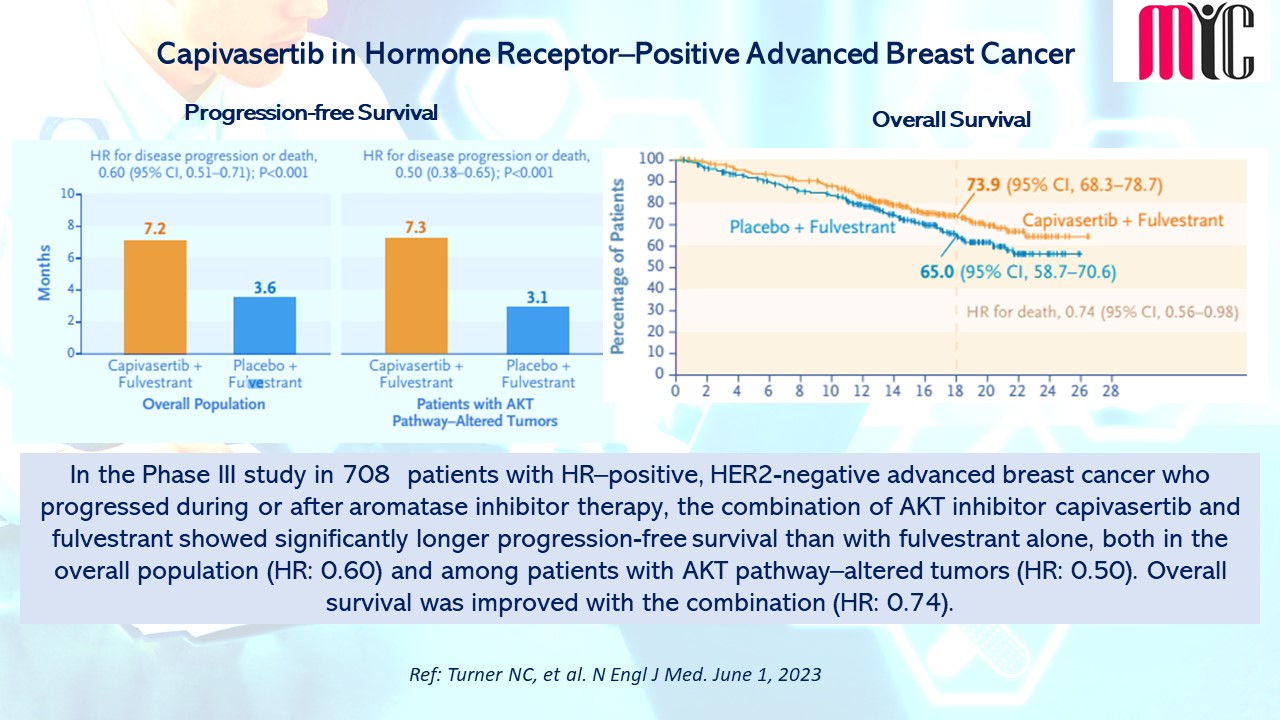
In the Phase III study in 708 patients with HR–positive, HER2-negative advanced breast cancer who progressed during or after aromatase inhibitor therapy, the combination of AKT inhibitor capivasertib and fulvestrant showed significantly longer progression-free survival than with fulvestrant alone, both in the overall population (HR: 0.60) and among patients with AKT pathway–altered tumors (HR: 0.50). Overall survival was improved with the combination (HR: 0.74). (Ref: Turner NC, et al. N Engl J Med. June 1, 2023)
#oncologyresearch #clinicalresearch #clinicaldevelopment




Sorry, the comment form is closed at this time.