02 May Dostarlimab for Primary Advanced or Recurrent Endometrial Cancer
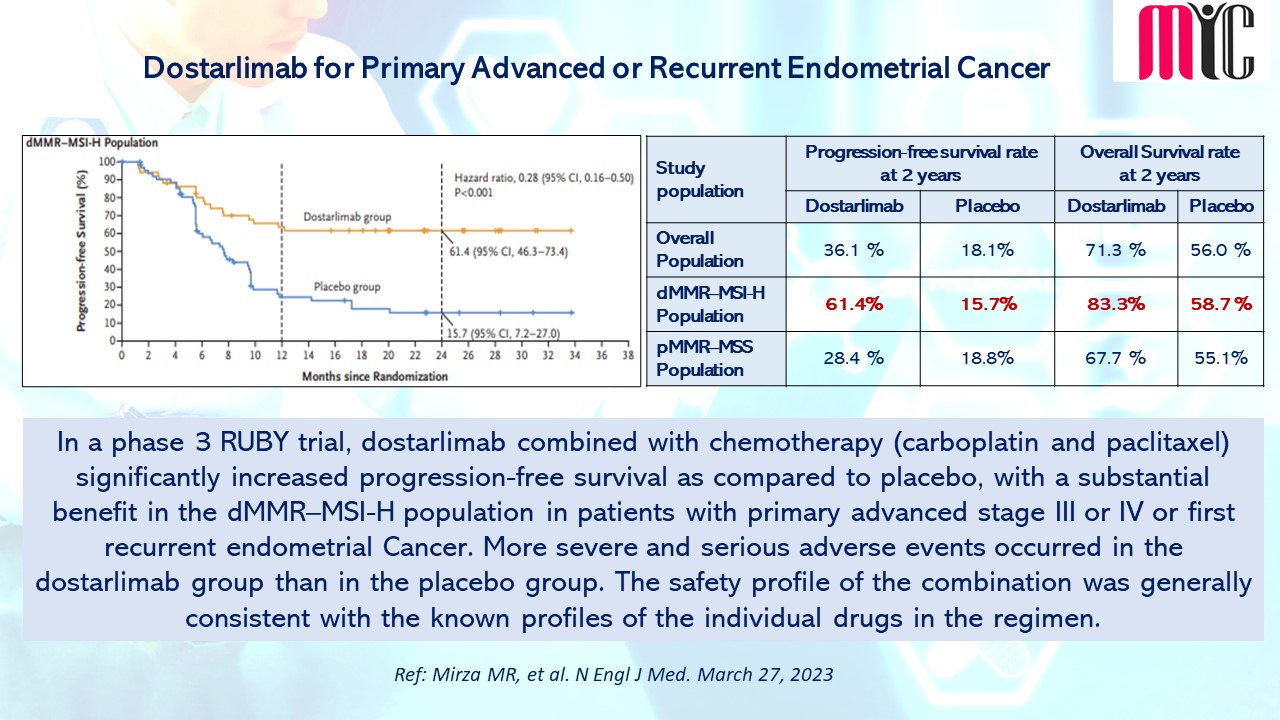
In a phase 3 RUBY trial, dostarlimab combined with chemotherapy (carboplatin and paclitaxel) significantly increased progression-free survival as compared to placebo, with a substantial benefit in the dMMR–MSI-H population in patients with primary advanced stage III or IV or first recurrent endometrial Cancer. More severe and serious adverse events occurred in the dostarlimab group than in the placebo group. The safety profile of the combination was generally consistent with the known profiles of the individual drugs in the regimen. (Ref: Mirza MR, et al. N Engl J Med. March 27, 2023)
#oncologyresearch #clinicalresearch #clinicaldevelopment




Sorry, the comment form is closed at this time.