30 Apr Ivosidenib and Azacitidine combination shows significant clinical benefit in newly diagnosed IDH1-mutated AML
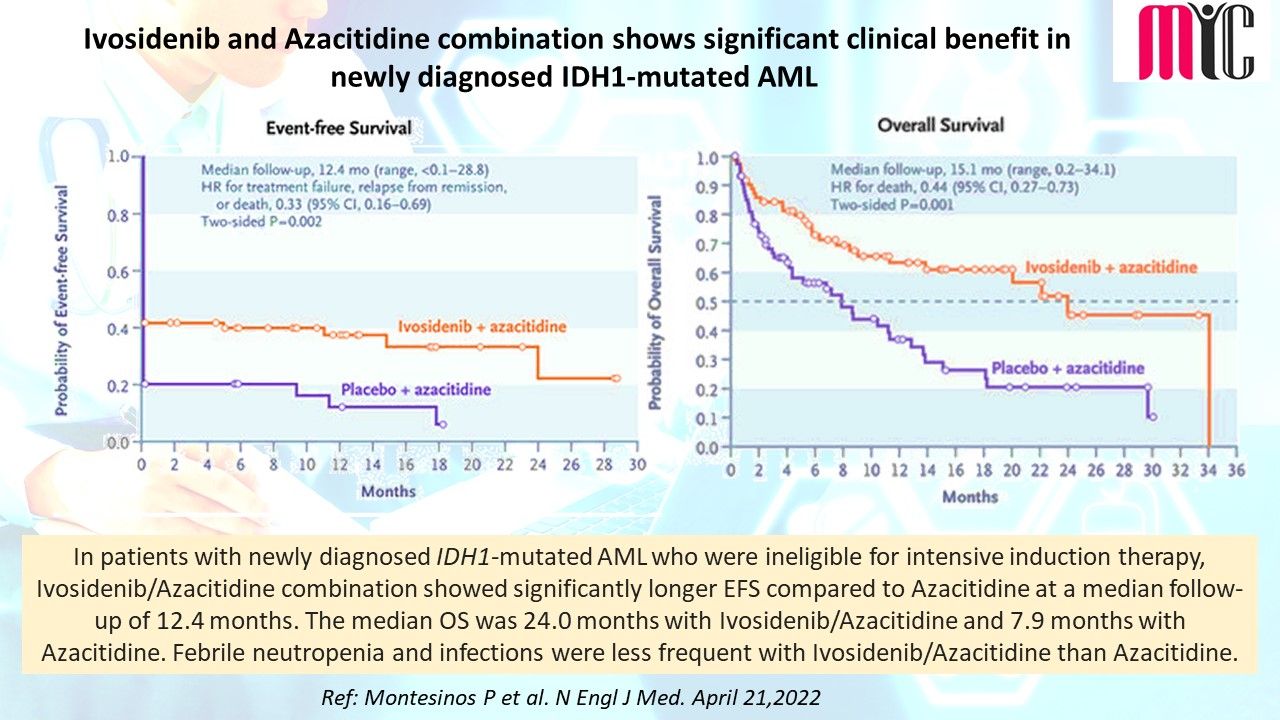
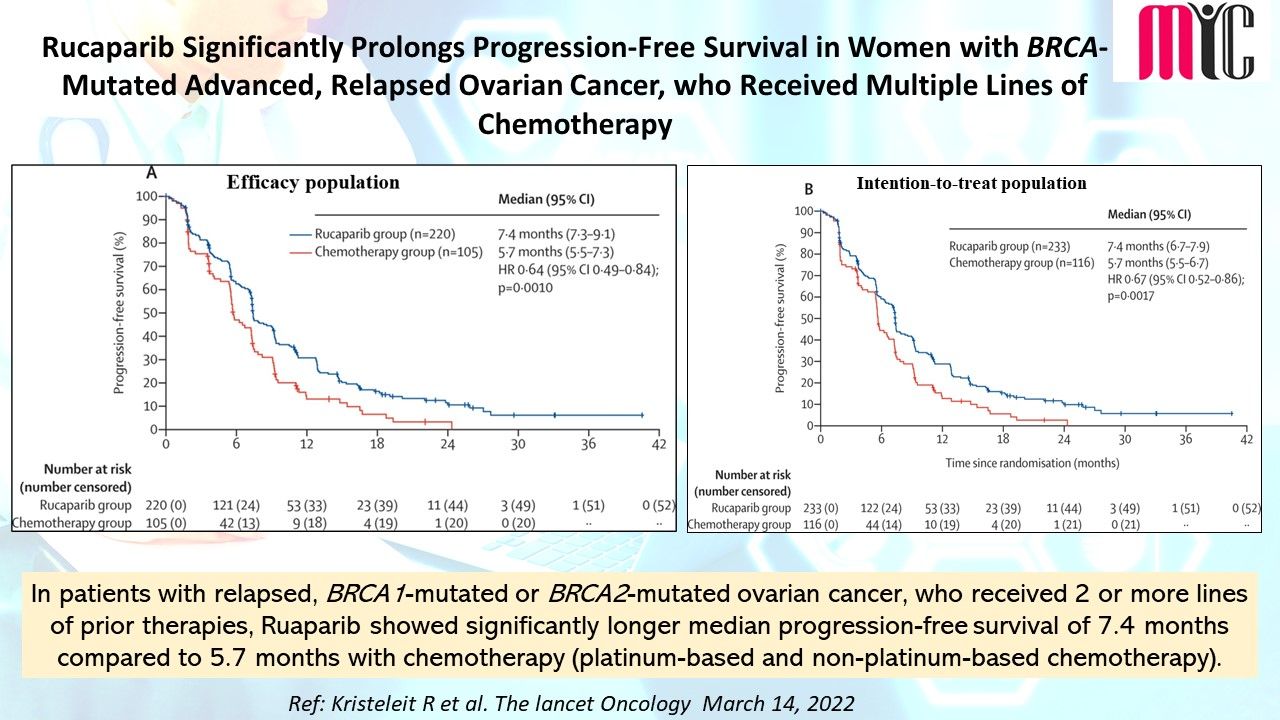
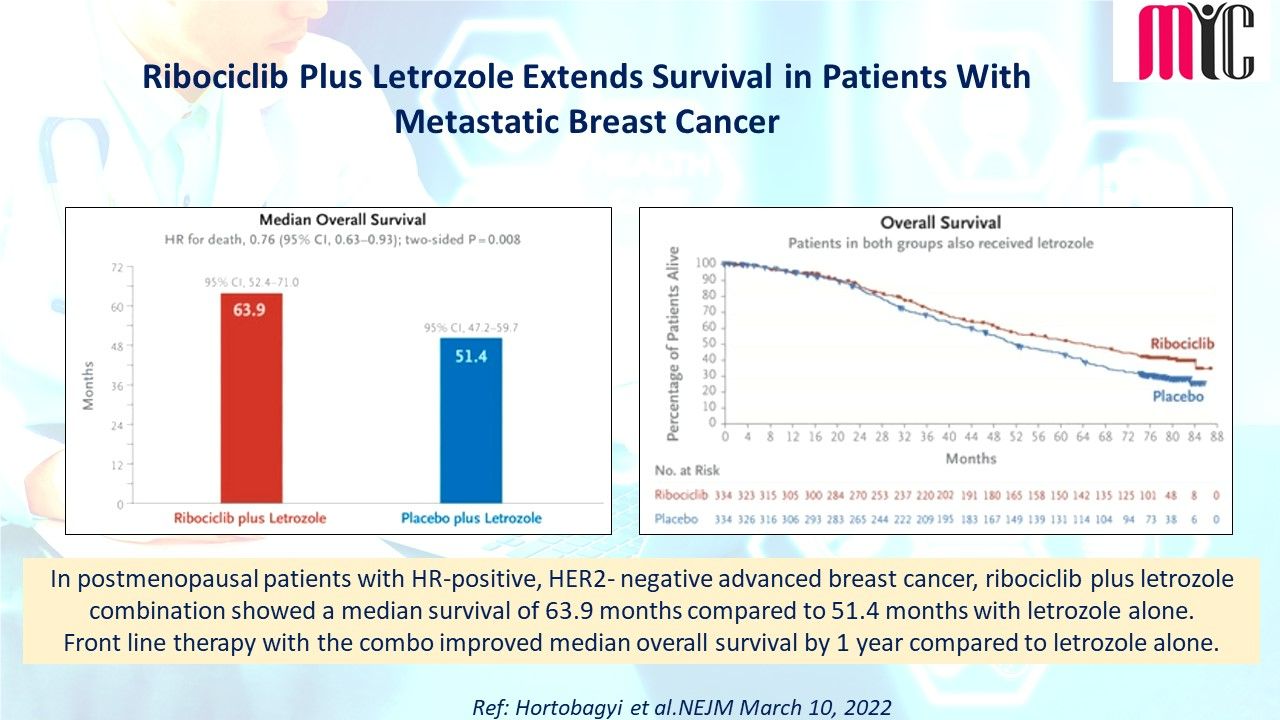
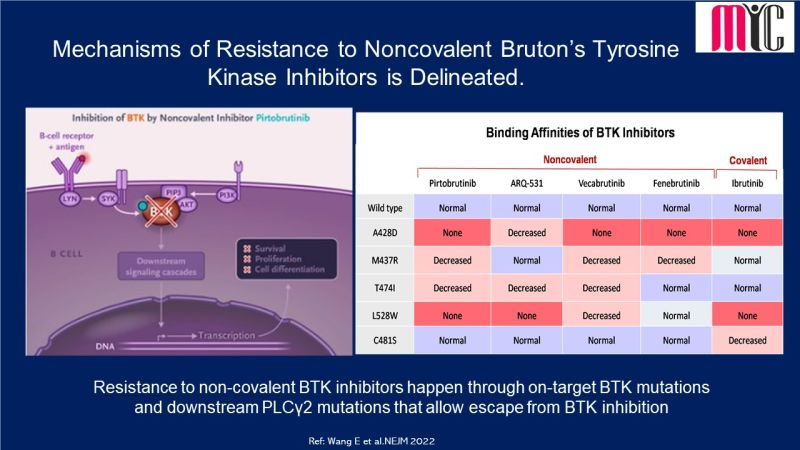
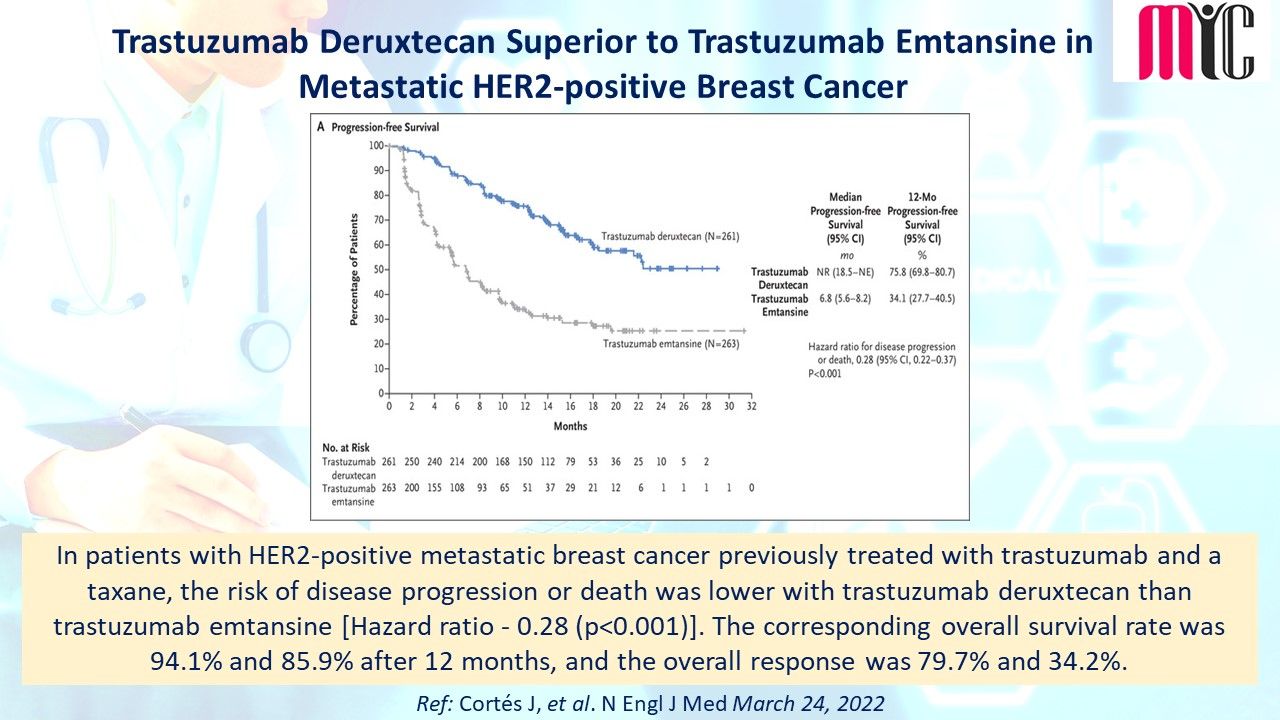
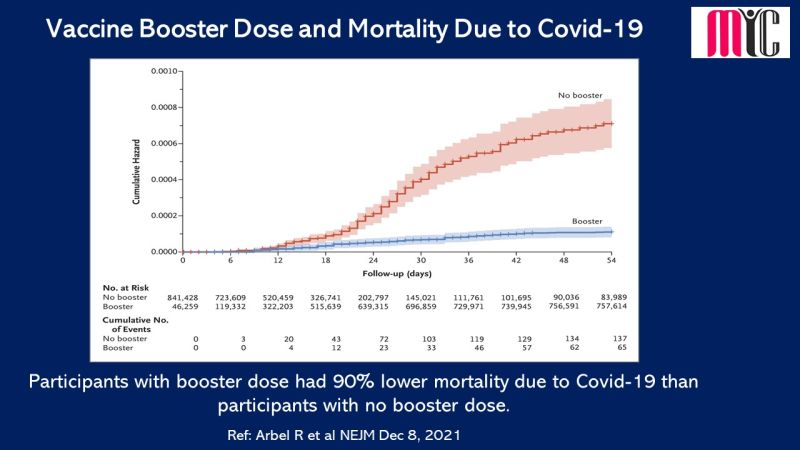
n patients with newly diagnosed IDH1-mutated AML who were ineligible for intensive induction therapy, Ivosidenib/Azacitidine combination showed significantly longer EFS compared to Azacitidine at a median follow-up of 12.4 months. The median OS was 24.0 months with Ivosidenib/Azacitidine and 7.9 months with Azacitidine. Febrile neutropenia and infections were less frequent with Ivosidenib/Azacitidine than Azacitidine.
(Ref: Montesinos P et al. N Engl J Med. April 21,2022)
#oncologyresearch #drugdevelopment
https://www.linkedin.com/feed/update/urn:li:activity:6926072382784704512