02 Oct Oncology Drug Development – FDA Suggests New Trial Designs for Accelerated Approvals
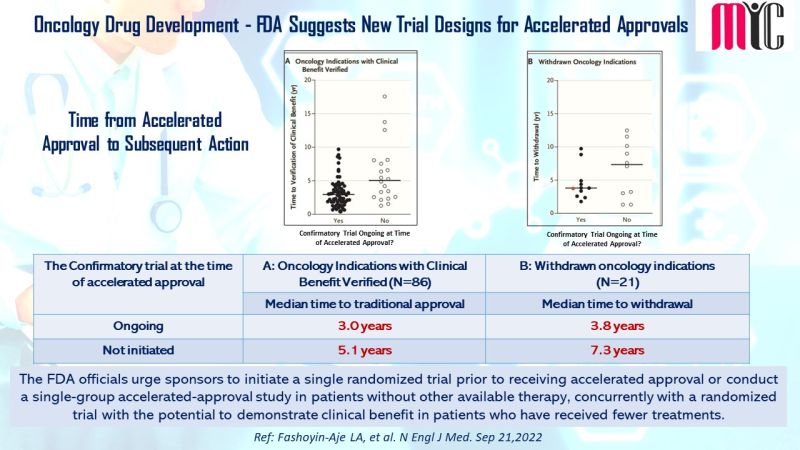
The FDA officials urge sponsors to initiate a single randomized trial prior to receiving accelerated approval or conduct a single-group accelerated-approval study in patients without other available therapy, concurrently with a randomized trial with the potential to demonstrate clinical benefit in patients who have received fewer treatments.
(Ref: Fashoyin-Aje LA et al, N Engl J Med. Sep 21,2022)
#oncologyresearch #oncologytrials #drugdevelopment #clinicalresearch #fdaapproval # #fda
https://www.linkedin.com/feed/update/urn:li:activity:6982350995125092352




Sorry, the comment form is closed at this time.