12 Aug Erdafitinib in patients with advanced solid tumours with FGFR alterations
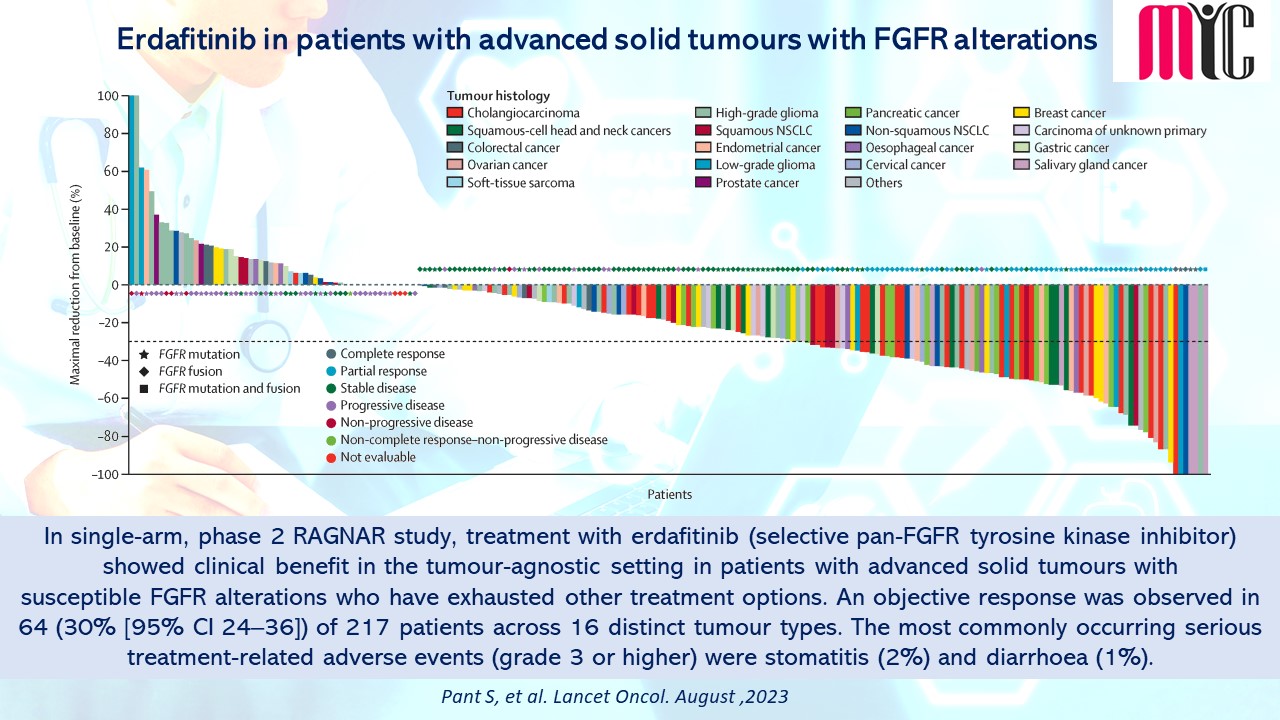
In single-arm, phase 2 RAGNAR study, treatment with erdafitinib (selective pan-FGFR tyrosine kinase inhibitor) showed clinical benefit in the tumour-agnostic setting in patients with advanced solid tumours with susceptible FGFR alterations who have exhausted other treatment options. An objective response was observed in 64 (30% [95% CI 24–36]) of 217 patients across 16 distinct tumour types. The most commonly occurring serious treatment-related adverse events (grade 3 or higher) were stomatitis (2%) and diarrhoea (1%). (Pant S, et al. Lancet Oncol. August 2023)




Sorry, the comment form is closed at this time.