07 Sep Combination of Ceralasertib and Olaparib in Acquired PARP-Inhibitor-Resistant HR Deficient Ovarian Cancer
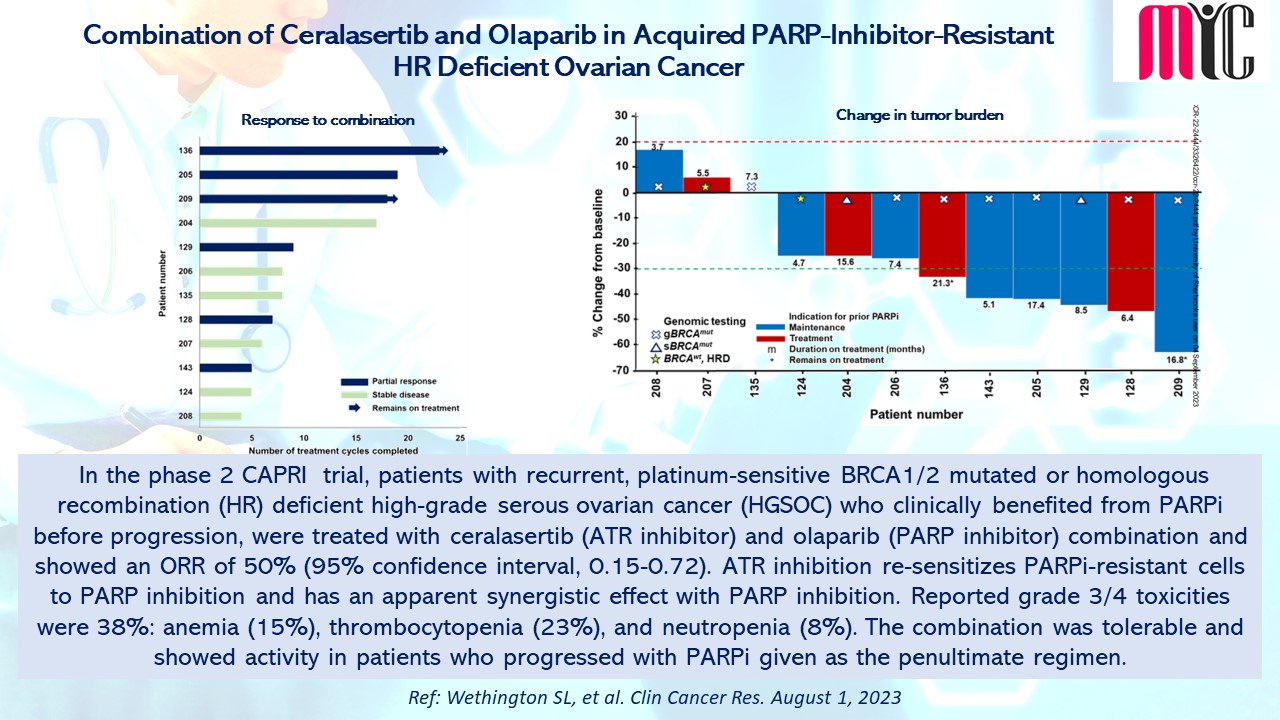
In the phase 2 CAPRI trial, patients with recurrent, platinum-sensitive BRCA1/2 mutated or homologous recombination (HR) deficient high-grade serous ovarian cancer (HGSOC) and clinically benefited from PARPi before progression, treated with ceralasertib (ATR inhibitor) and olaparib (PARP inhibitor) showed ORR of 50% (95% confidence interval, 0.15-0.72). ATR inhibition re-sensitizes PARPi-resistant cells to PARP inhibition and has an apparent synergistic effect with PARP inhibition. Grade 3/4 toxicities were 38%: anemia (15%), thrombocytopenia (23%), and neutropenia (8%). The combination was tolerable and showed activity in patients who progressed with PARPi as the penultimate regimen. (Ref: Wethington SL, et al. Clin Cancer Res. August 1, 2023)
#oncologyresearch #clinicalresearch #clinicaldevelopment




Sorry, the comment form is closed at this time.