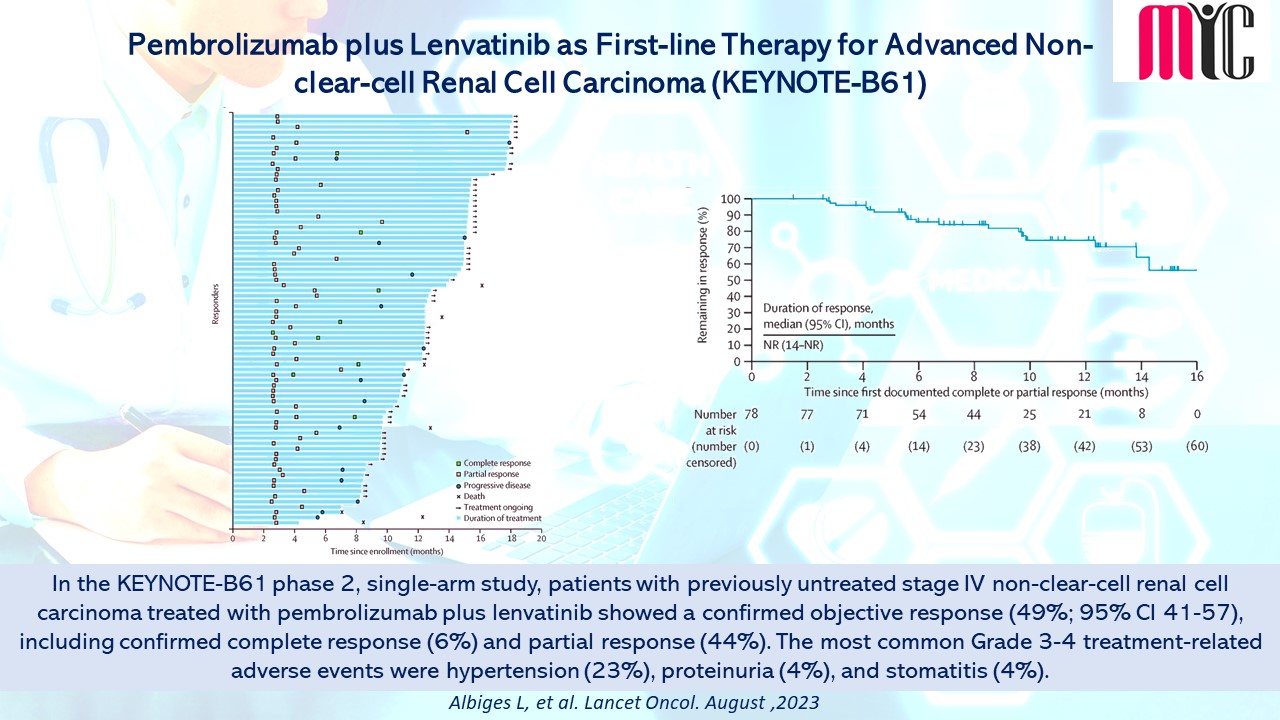
16 Aug Pembrolizumab plus Lenvatinib as First-line Therapy for Advanced Non-clear-cell Renal Cell Carcinoma (KEYNOTE-B61)
In the KEYNOTE-B61 phase 2, single-arm study, patients with previously untreated stage IV non-clear-cell renal cell carcinoma treated with pembrolizumab plus lenvatinib showed a confirmed objective response (49%; 95% CI 41-57), including confirmed complete response (6%) and partial response (44%). The most common Grade 3-4 treatment-related adverse events were hypertension (23%), proteinuria (4%), and stomatitis (4%). (Albiges L, et al. Lancet Oncol. August, 2023)
#oncologyresearch #clinicalresearch #clinicaldevelopment