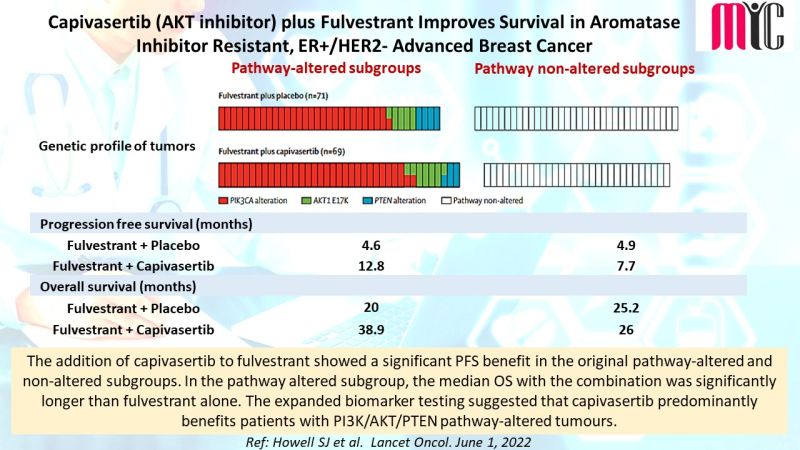
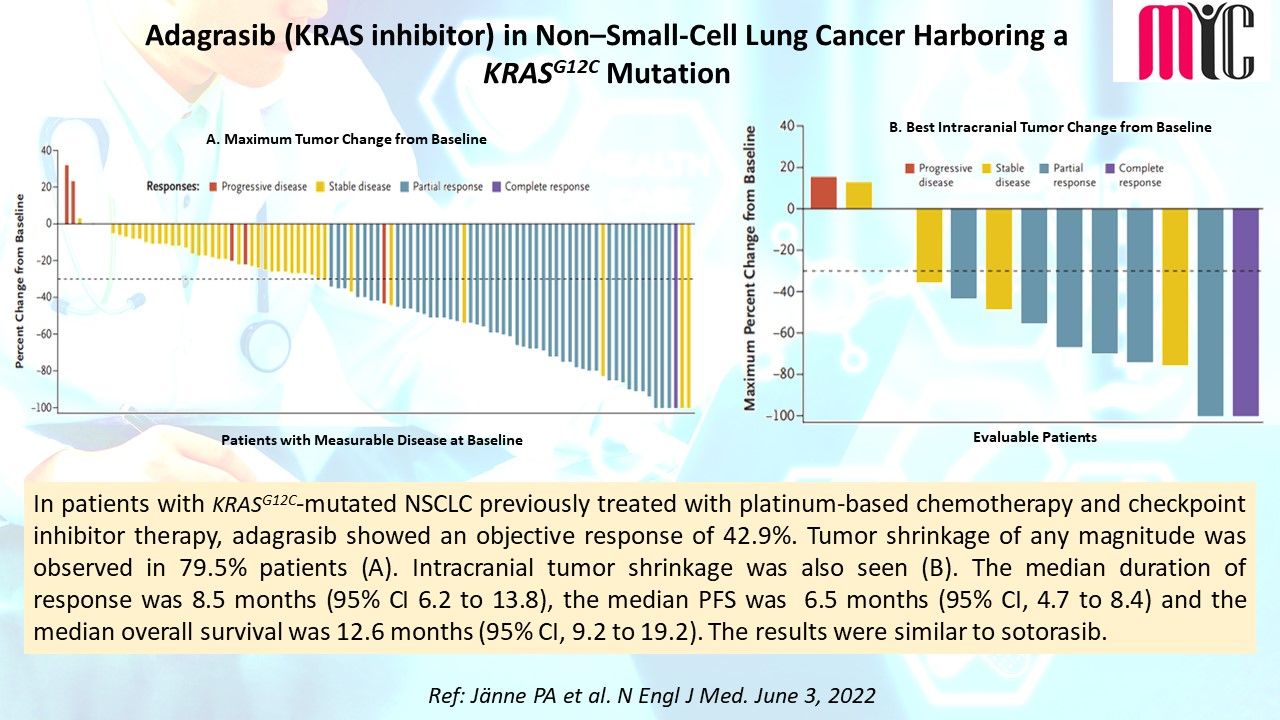
18 Jun Capivasertib (AKT inhibitor) plus Fulvestrant Improves Survival in Aromatase Inhibitor Resistant, ER+/HER2- Advanced Breast Cancer
The addition of capivasertib to fulvestrant showed a significant PFS benefit in the original pathway-altered and non-altered subgroups. In the pathway altered subgroup, the median OS with the combination was significantly longer than fulvestrant alone. The expanded biomarker testing suggested that capivasertib predominantly benefits patients with PI3K/AKT/PTEN pathway-altered tumours. (Ref: Howell SJ et al. Lancet Oncol. June 1, 2022)
#oncologyresearch #clinicaldevelopment
https://www.linkedin.com/feed/update/urn:li:activity:6943811898039484416