28 Nov FDA Approves Tremelimumab in Combination With Durvalumab and Platinum-based Chemotherapy for Metastatic Non-small Cell Lung Cancer (NSCLC)
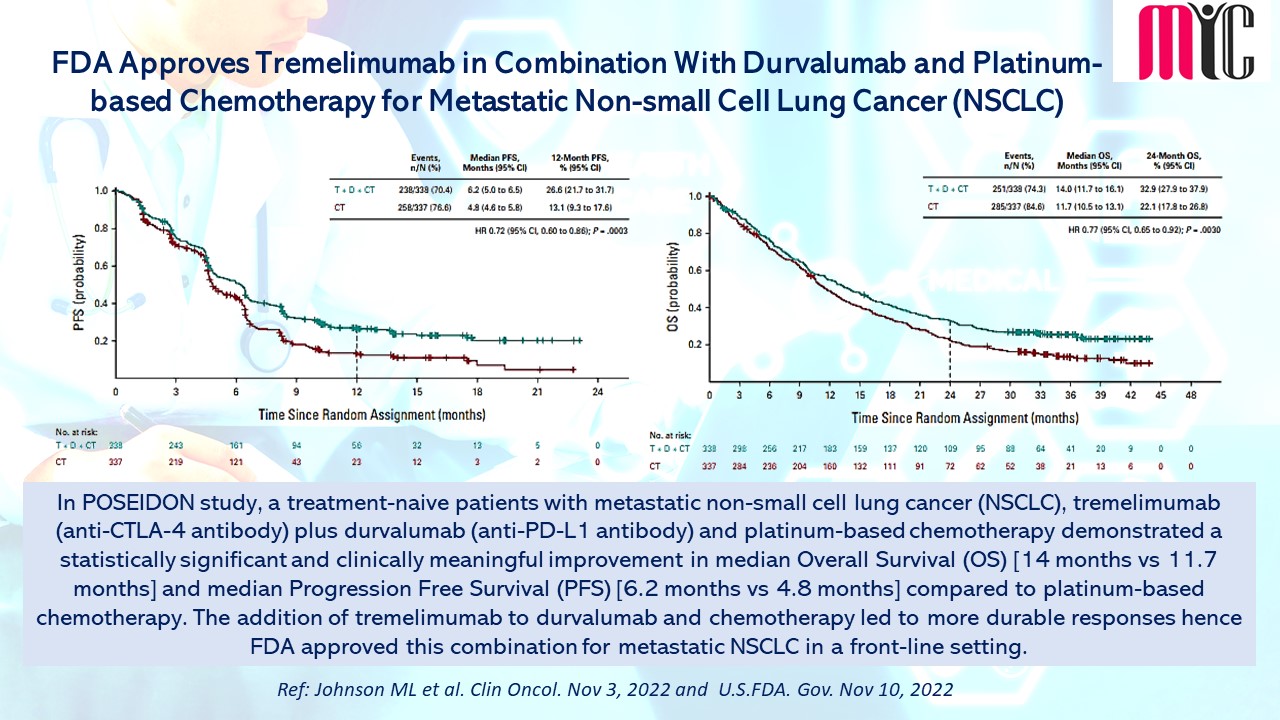
In POSEIDON study, a treatment-naive patients with metastatic non-small cell lung cancer (NSCLC), tremelimumab (anti-CTLA-4 antibody) plus durvalumab (anti-PD-L1 antibody) and platinum-based chemotherapy demonstrated a statistically significant and clinically meaningful improvement in median Overall Survival (OS) [14 months vs 11.7 months] and median Progression Free Survival (PFS) [6.2 months vs 4.8 months] compared to platinum-based chemotherapy. The addition of tremelimumab to durvalumab and chemotherapy led to more durable responses hence FDA approved this combination for metastatic NSCLC in a front-line setting. (Ref: Johnson ML et al. Clin Oncol. Nov 3, 2022 and U.S.FDA. Gov. Nov 10, 2022)
#oncologyresearch #clinicalresearch #clinicaldevelopment




Sorry, the comment form is closed at this time.