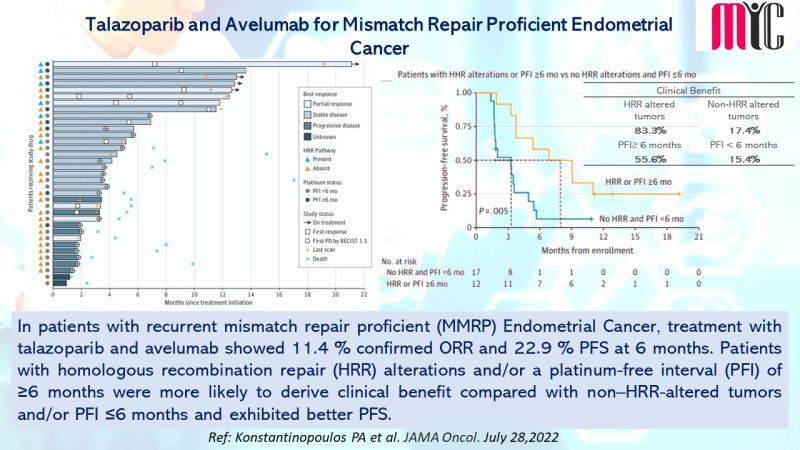
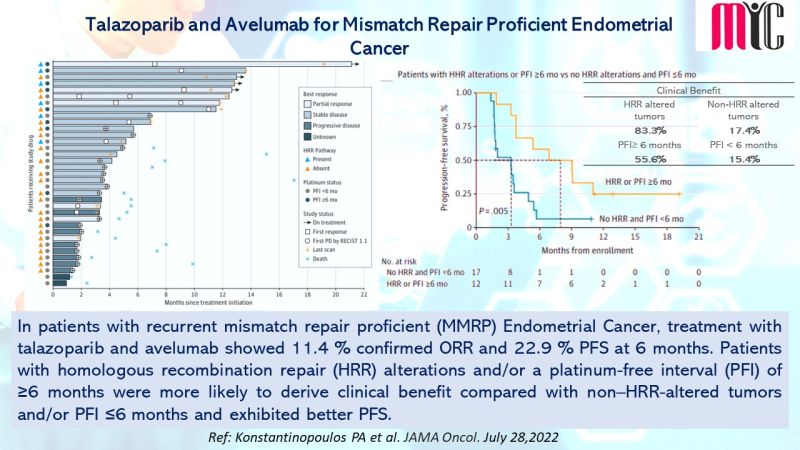
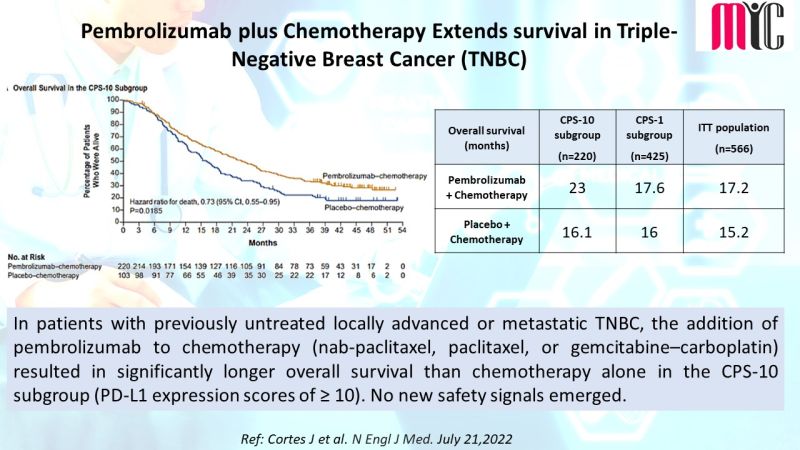
27 Aug Ripretinib Exhibits Better Tolerability Profile than Sunitinib in Advanced GIST Tumors
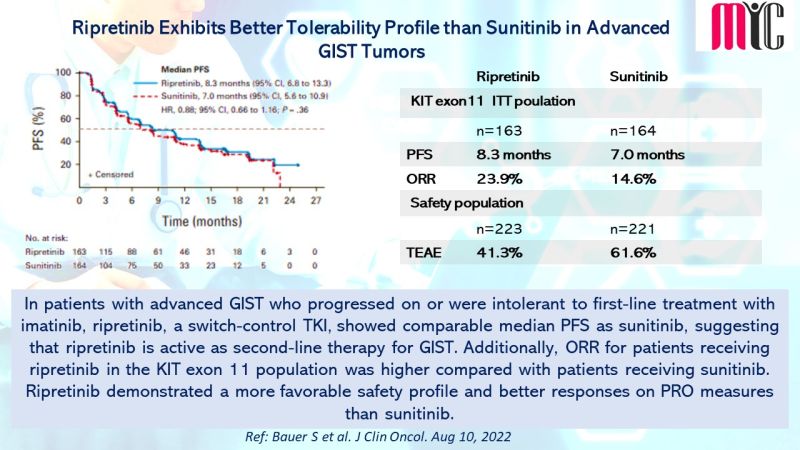
In patients with advanced GIST who progressed on or were intolerant to first-line treatment with imatinib, ripretinib, a switch-control TKI, showed comparable median PFS as sunitinib, suggesting that ripretinib is active as second-line therapy for GIST. Additionally, ORR for patients receiving ripretinib in the KIT exon 11 population was higher compared with patients receiving sunitinib. Ripretinib demonstrated a more favorable safety profile and better responses on PRO measures than sunitinib. (Ref: Bauer S et al. J Clin Oncol. Aug 10, 2022)
#oncologyresearch #clinicalresearch #clinicaldevelopment
https://www.linkedin.com/feed/update/urn:li:activity:6969173938740310016